New nanostructure for batteries keeps going and going
(Phys.org) -- For more than a decade, scientists have tried to improve lithium-based batteries by replacing the graphite in one terminal with silicon, which can store 10 times more charge. But after just a few charge/discharge cycles, the silicon structure would crack and crumble, rendering the battery useless.
Now a team led by materials scientist Yi Cui of Stanford and SLAC has found a solution: a cleverly designed double-walled nanostructure that lasts more than 6,000 cycles, far more than needed by electric vehicles or mobile electronics.
“This is a very exciting development toward our goal of creating smaller, lighter and longer-lasting batteries than are available today,” Cui said. The results were published March 25 in Nature Nanotechnology.
Lithium-ion batteries are widely used to power devices from electric vehicles to portable electronics because they can store a relatively large amount of energy in a relatively lightweight package. The battery works by controlling the flow of lithium ions through a fluid electrolyte between its two terminals, called the anode and cathode.
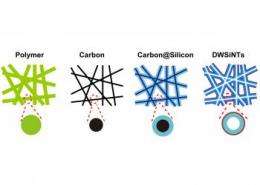
The promise – and peril – of using silicon as the anode in these batteries comes from the way the lithium ions bond with the anode during the charging cycle. Up to four lithium ions bind to each of the atoms in a silicon anode – compared to just one for every six carbon atoms in today’s graphite anode – which allows it to store much more charge.
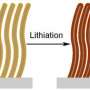
However, it also swells the anode to as much as four times its initial volume. What’s more, some of the electrolyte reacts with the silicon, coating it and inhibiting further charging. When lithium flows out of the anode during discharge, the anode shrinks back to its original size and the coating cracks, exposing fresh silicon to the electrolyte.
Within just a few cycles, the strain of expansion and contraction, combined with the electrolyte attack, destroys the anode through a process called "decrepitation."
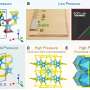
Over the past five years, Cui’s group has progressively improved the durability of silicon anodes by making them out of nanowires and then hollow silicon nanoparticles. His latest design consists of a double-walled silicon nanotube coated with a thin layer of silicon oxide, a very tough ceramic material.
This strong outer layer keeps the outside wall of the nanotube from expanding, so it stays intact. Instead, the silicon swells harmlessly into the hollow interior, which is also too small for electrolyte molecules to enter. After the first charging cycle, it operates for more than 6,000 cycles with 85 percent capacity remaining.
Cui said future research is aimed at simplifying the process for making the double-wall silicon nanotubes. Others in his group are developing new high-performance cathodes to combine with the new anode to form a battery with five times the performance of today’s lithium-ion technology.
In 2008, Cui founded a company, Amprius, which licensed rights to Stanford’s patents for his silicon nanowire anode technology. Its near-term goal is to produce a battery with double the energy density of today’s lithium-ion batteries.
Provided by SLAC National Accelerator Laboratory