Defensive symbiosis leads to gene loss in bacterial partners

Antibiotics on the cocoon protect the offspring of beewolves, a group of digger wasps, from detrimental fungi. These protective substances are produced by symbiotic bacteria of the genus Streptomyces, which live in these insects. In a new study in PNAS, researchers from the Max Planck Institute for Chemical Ecology and the University of Mainz, together with an international team, showed that these beneficial bacteria are losing genetic material that is no longer needed. The genome of these bacteria is of great interest for understanding the process of genome erosion and elucidating how the cooperation and the mutual benefit between bacteria and their host insects have evolved over long periods of time.
An ancient defensive symbiosis ensures the survival of the beewolves' offspring
Beewolves of the genus Philanthus belong to the digger wasp family and—as the name suggests—hunt bees as a food source for their offspring. They dig holes in the ground, bury their prey and lay their eggs. To protect their young from mold fungi in the warm and moist conditions in the soil, female beewolves secrete a substance from their antennae that contains symbiotic bacteria of the genus Streptomyces. These produce a cocktail of various antibiotic substances, which the beewolf larvae spin into their cocoon. This defensive symbiosis, which has existed for more than 68 million years, ensures that the beewolf offspring is well protected against harmful microorganisms.
Signs of genome erosion in the bacterial partner
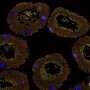
The genome of the bacteria Streptomyces philanthi that are associated with the European beewolf Philanthus triangulum has now been studied in detail by a team led by Martin Kaltenpoth, director of the new Department of Insect Symbiosis at the Max Planck Institute for Chemical Ecology. "We wondered whether the long-term association with the host has resulted in changes in the symbiont's genome or shaped gene regulation and metabolic interactions between the beewolf and its bacterial partners," explains Mario Sandoval-Calderón, one of the first authors of the study, the motivation for this study.
Using state-of-the-art gene sequencing techniques, the researchers were able to read out the complete genome of the symbiont. They noticed the accumulation of "pseudogenes" that arise as a result of a reading frame shift of the coding base pairs. "These frameshift mutations in probably inactivated genes are indicators of an incipient genome erosion in Streptomyces philanthi. While the outcome of genome erosion is well characterized, its onset and the early stages are less well understood. Thus, having access to an organism in the initial stage of genomic decay can help us understand how the process starts," says study leader Martin Kaltenpoth.
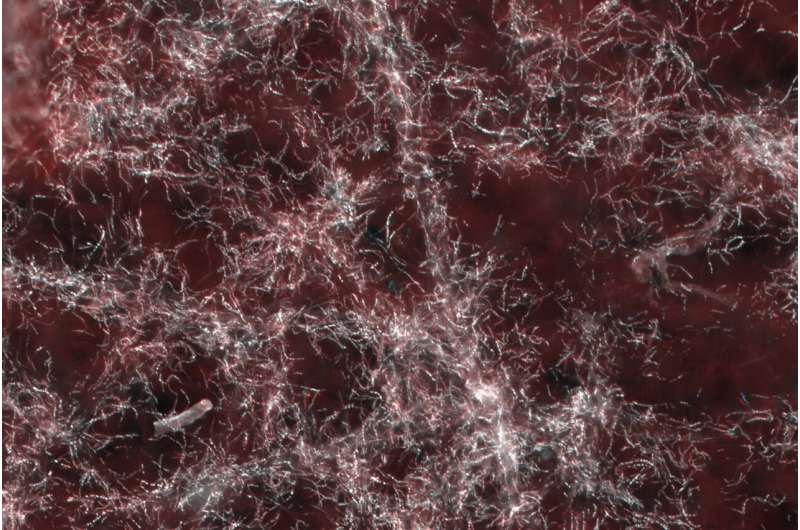
Genes: reduced to the defensive symbiosis?
Further genetic analyses provided hints that the metabolism of the bacterial symbionts is mainly directed towards the production of antibiotic substances necessary for the protection of the beewolves' offspring. The unresolved question why the antibiotics are also produced in the antennae of female beewolves and then released into the brood cells via secretion of a substance containing the bacteria and the antibiotic cocktail could not be answered. Their actual defensive function has so far only been demonstrated on the cocoon. Instead, the analyses provided evidence of certain amino acids that the host must supply to its symbiotic partners in the antennal gland reservoirs because Streptomyces philanthi bacteria are no longer able to produce these nutrients themselves. Thus, is seems that beewolves even have control over antibiotic production and are able to self-regulate the benefits provided by their bacterial partners in order to optimize the protection of their offspring. Further analyses are necessary to confirm this hypothesis.
The observation that the genomic decay in the symbionts appears to be in its early stages is surprising after such a long defensive alliance with beewolves. Therefore, the scientists are now also investigating the genomes of symbiont strains associated with other beewolf species. "It seems possible that only some strains are experiencing genome erosion. Understanding the reasons for this and the factors that initiate this process could help us to gain valuable insights into the forces that govern genome evolution more generally," Martin Kaltenpoth hopes.
Modern molecular tools make it increasingly possible to study the interactions between living organisms from the level of individual molecules to ecological and evolutionary questions—even in non-model organisms. Applying them to the manifold symbioses between insects and their bacterial partners may help to better understand the interactions between these organisms, their mutual benefits as well as their co-evolution.
More information: Taras Y. Nechitaylo el al., "Incipient genome erosion and metabolic streamlining for antibiotic production in a defensive symbiont," PNAS (2021). www.pnas.org/cgi/doi/10.1073/pnas.2023047118
Journal information: Proceedings of the National Academy of Sciences
Provided by Max Planck Society