This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Efficient synthesis of indole derivatives, an important component of most drugs, allows the development of new drugs
A research group at Nagoya University in Japan has successfully developed an ultrafast and simple synthetic method for producing indole derivatives. Their findings are expected to make drug production more efficient and increase the range of potential indole-based pharmaceuticals to treat a variety of diseases. Their findings were published in Communications Chemistry.
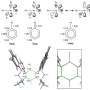
An indole is an organic compound consisting of a benzene ring and a pyrrole ring. Heteroatom alkylation at the carbon atom next the indole ring is particularly useful to create wide range of new indole derivatives and many anti-inflammatory, anticancer, and antimicrobial treatments contain them
In the past, this heteroatom alkylation has proven difficult because indoles easily and rapidly undergo unwanted dimerization/multimerization, processes in which two or more molecules combine during the reaction to form unwanted larger molecules. These unwanted by-products limit the yield of the desired product.
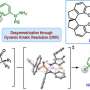
Since indoles are common to so many drugs, an efficient method of synthesizing them is essential. Now, a team consisting of Assistant Professor Hisashi Masui (he/him), graduate student Sena Kanda (she/her), and Professor Shinichiro Fuse (he/him) at the Graduate School of Pharmaceutical Sciences, Nagoya University, obtained the target indole-based product in high yield while limiting side reactions using a new microflow synthesis method.
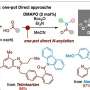
Their method consists of flowing a solution through a small channel with an inner diameter of about 1 mm. Because of its small size, the channel has a high surface-area-to-volume ratio, allowing the solution to be mixed in a few milliseconds. This allows for precise control of short reaction times and limits the time when unstable intermediates are present in the reaction process to only about 0.1 seconds. This is fast enough to prevent unwanted dimerization/multimerization.
"Although it takes several seconds to mix solutions using a flask, the microflow synthesis method does it in less than a few milliseconds," Fuse said.
"Therefore, short reaction times of less than a second can be generated and more precisely controlled and the reaction temperature can be fully controlled. We created an activated indole compound in 20 ms to achieve a target product yield of 95%. When the same reaction was performed in a standard flask, the target product could not be obtained at all, and side reactions occurred during the mixing of the solutions."
"Since the developed method can be used to synthesize various indole derivatives, our study is useful for creating drug candidates and improving the efficiency of drug production," said Fuse. "The reaction proceeds at an extremely high speed under mild room temperature conditions, and the reactants used are readily available and inexpensive, making it highly practical."
The group sees many potential industrial applications. Fuse emphasized that the flow synthesis method can be reproducibly scaled up by continuous pumping, making it an ideal choice for manufacturing facilities. "Indoles are one of the most abundant structures in pharmaceuticals," he said. "We expect it to contribute to the creation of drug candidates and the efficiency of pharmaceutical production."
More information: Hisashi Masui et al, Verification of preparations of (1H-indol-3-yl)methyl electrophiles and development of their microflow rapid generation and substitution, Communications Chemistry (2023). DOI: 10.1038/s42004-023-00837-1
Provided by Nagoya University