This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A divergent strategy enables molecular diversity: Synthesis of nine complex natural compounds
A research group led by chemist Thomas Magauer has accomplished a divergent strategy to synthesize nine complex natural compounds. The developed method requires significantly less time and results in a variety of compounds with different structures and biological properties. The paper is published in the journal Angewandte Chemie International Edition.
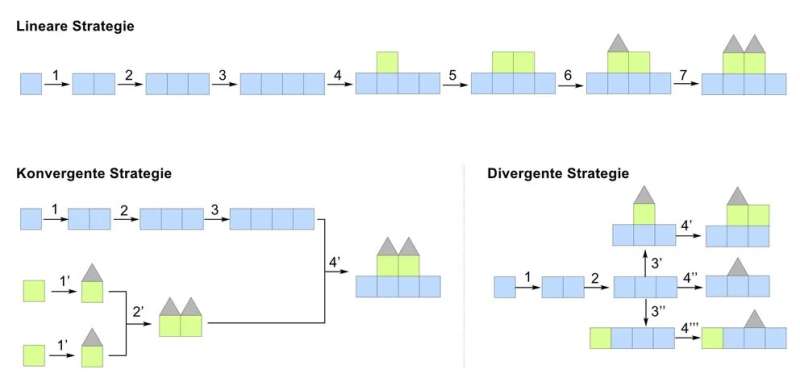
Chemical synthesis enables the construction of complex molecules and active drug substances. Various strategies based on linear, convergent or divergent synthesis can be employed. For linear strategies, a target compound is synthesized from a single starting material, similar to building a house by assembling individual bricks. In contrast, convergent approaches are analogous to prefabricated houses, where smaller molecular units are linked together to form a larger molecule at a later stage of the synthesis. For both strategies, the target compound possesses the same molecular structure.
Mimicking nature's strategies
In nature, functionalization or rearrangement of a common molecular structures allows for the modification of the biological activity. Such structurally related compounds are of great importance in drug research. However, unlike in nature, adjustment of the starting materials and iteration of the synthesis sequence are usually necessary in the chemical laboratory. Thus, the synthetic generation of structural diversity through linear and convergent strategies in the lab is time-consuming.
The research group of Thomas Magauer, Professor of Synthesis and Synthetic Methods at the University of Innsbruck, has now developed a divergent strategy to address this problem. Starting from a common advanced molecular intermediate, different reaction pathways are performed, resulting in structural variations in only one step. This type of synthesis requires significantly less time and leads to a variety of compounds with different molecular structures and biological properties.
Synthesis of nine complex compounds
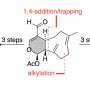
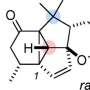
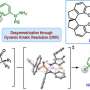
The divergent strategy allowed the research group to access nine complex indole sesquiterpene natural compounds: greenwayodendrines, greenwaylactams, polysins, and polyveolins. Indole sesquiterpenes are secondary metabolites found in plants, fungi, and insects, which are biosynthetically derived from an indole subunit (a bicyclic aromatic heterocycle) and a linear 15-membered carbon chain.
In nature, the assembly of the basic frameworks starts from such linear precursors through a divergent cyclization reaction. Subsequent oxidative modifications then complete the structure of indole sesquiterpenes, which are crucial for the biological activity and stability of each molecule.
The synthesis of the linear precursor in the laboratory proved to be the easiest task for the group. However, the selective initiation of different cyclization reactions to form the desired ring structures was much more difficult. Such cyclizations have been strictly limited to carbon terminations of the indole framework. Although an alternative nitrogen-based cyclization had been postulated in the biosynthesis of certain natural compounds, considerations for its realization in the laboratory had failed due to the increased reactivity of the C3 position.
The targeted and reversible blocking of the C3 position allows for the realization of this termination for the first time and gave access to four different molecular architectures. In analogy to the biosynthesis, these architectures could be converted into the corresponding natural compounds through selective post-modifications. In summary, the developed sequence is characterized by high divergence and thus structural diversity. A total of nine natural compounds were synthesized in only five to eight steps, with an overall yield of up to 32%.
More information: Immanuel Plangger et al, A Divergent Polyene Cyclization for the Total Synthesis of Greenwayodendrines, Greenwaylactams, Polysin and Polyveoline, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202307719
Journal information: Angewandte Chemie International Edition
Provided by University of Innsbruck