This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Graphene discovery could help generate cheaper and more sustainable hydrogen
Researchers from The University of Manchester and the University of Warwick finally solved the long-standing puzzle of why graphene is so much more permeable to protons than expected by theory.
A decade ago, scientists at The University of Manchester demonstrated that graphene is permeable to protons, nuclei of hydrogen atoms. The unexpected result started a debate in the community because theory predicted that it would take billions of years for a proton to permeate through graphene's dense crystalline structure. This had led to suggestions that protons permeate not through the crystal lattice itself, but through the pinholes in its structure.
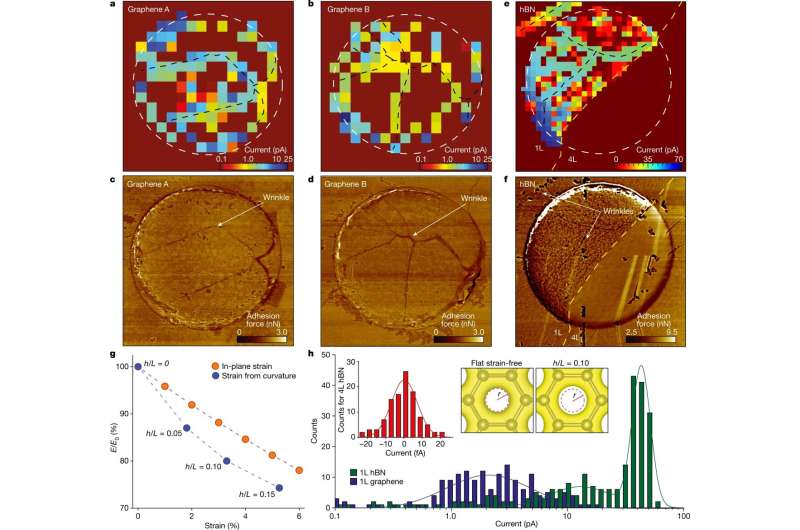
Now, writing in Nature, a collaboration between the University of Warwick, led by Prof Patrick Unwin, and The University of Manchester, led by Dr. Marcelo Lozada-Hidalgo and Prof Andre Geim, report ultra-high spatial resolution measurements of proton transport through graphene and prove that perfect graphene crystals are permeable to protons. Unexpectedly, protons are strongly accelerated around nanoscale wrinkles and ripples in the crystal.
The discovery has the potential to accelerate the hydrogen economy. Expensive catalysts and membranes, sometimes with significant environmental footprint, currently used to generate and utilize hydrogen could be replaced with more sustainable 2D crystals, reducing carbon emissions, and contributing to Net Zero through the generation of green hydrogen.
The team used a technique known as scanning electrochemical cell microscopy (SECCM) to measure minute proton currents collected from nanometer-sized areas. This allowed the researchers to visualize the spatial distribution of proton currents through graphene membranes. If proton transport took place through holes as some scientists speculated, the currents would be concentrated in a few isolated spots. No such isolated spots were found, which ruled out the presence of holes in the graphene membranes.
Drs Segun Wahab and Enrico Daviddi, leading authors of the paper, commented, "We were surprised to see absolutely no defects in the graphene crystals. Our results provide microscopic proof that graphene is intrinsically permeable to protons."
Unexpectedly, the proton currents were found to be accelerated around nanometer-sized wrinkles in the crystals. The scientists found that this arises because the wrinkles effectively 'stretch' the graphene lattice, thus providing a larger space for protons to permeate through the pristine crystal lattice. This observation now reconciles the experiment and theory.
Dr. Lozada-Hidalgo said, "We are effectively stretching an atomic scale mesh and observing a higher current through the stretched interatomic spaces in this mesh—mind-boggling."
Prof Unwin commented, "These results showcase SECCM, developed in our lab, as a powerful technique to obtain microscopic insights into electrochemical interfaces, which opens up exciting possibilities for the design of next-generation membranes and separators involving protons."
The authors are excited about the potential of this discovery to enable new hydrogen-based technologies.
Dr. Lozada-Hidalgo said, "Exploiting the catalytic activity of ripples and wrinkles in 2D crystals is a fundamentally new way to accelerate ion transport and chemical reactions. This could lead to the development of low-cost catalysts for hydrogen-related technologies."
More information: Marcelo Lozada-Hidalgo, Proton transport through nanoscale corrugations in two-dimensional crystals, Nature (2023). DOI: 10.1038/s41586-023-06247-6. www.nature.com/articles/s41586-023-06247-6
Journal information: Nature
Provided by University of Manchester