This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Evidence for reversible oxygen ion movement during electrical pulsing: Emerging ferroelectricity in binary oxides
Ferroelectric binary oxides thin films are garnering attention for their superior compatibility over traditional perovskite-based ferroelectric materials. Its compatibility and scalability within the CMOS framework make it an ideal candidate for integrating ferroelectric devices into mainstream semiconductor components, including next-generation memory devices and various logic devices such as Ferroelectric Field-effect Transistor, and Negative Capacitance Field-effect Transistor.
It has been reported that challenges remain in the widespread adoption of these materials, such as insufficient electrostatic control, compromised reliability, and serious variation for EOT scaling in terms of very large-scale integration.
Research published in Materials Futures has elucidated ferroelectric-type behaviors in amorphous dielectric film. However, it is hard to clearly distinguish this observed hysteresis and ferroelectricity with classic ferroelectric films with conclusive contributions of specific phases. Therefore, it is imperative to note that the classification of amorphous materials as ferroelectric is subject to ongoing scientific debate.
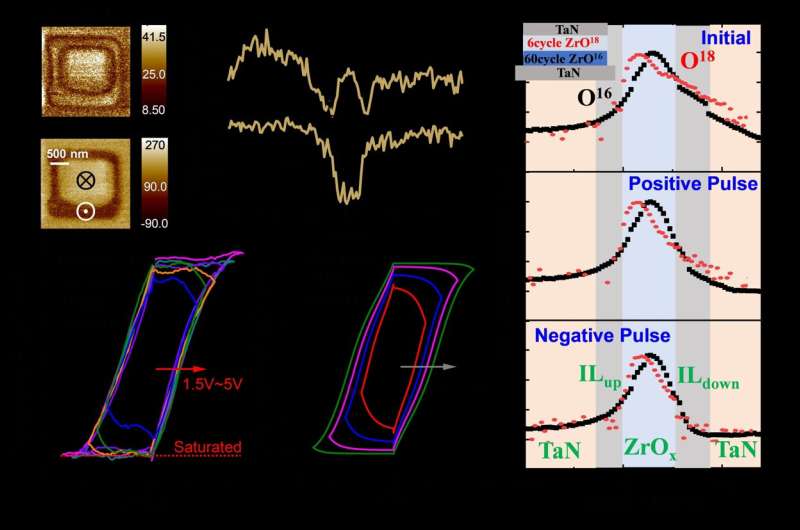
The physical mechanism for the ferroelectricity discussed by the authors involves the reversible movement of oxygen ions during electrical pulsing. This movement of oxygen ions is considered a key enabler for the emerging ferroelectric behavior observed in binary oxides. The authors suggest that this reversible oxygen ion movement plays a crucial role in inducing and controlling the ferroelectric properties of the materials.
The researchers found that emerging ferroelectricity exists in the ultrathin oxide system due to microscopic ion migration in the switching process. These ferroelectric binary oxide films are governed by the interface-limited switching mechanism. Nonvolatile memory devices featuring ultrathin amorphous dielectrics reduced operating voltage to ±1 V.
Although a series of characterization tests and simulation analyses have been conducted, the understanding of the mechanism behind the emerging ferroelectricity in amorphous dielectric remains limited. To advance the application of this novel ferroelectric material, further research on the theoretical mechanism must be carried out.
Prof. Yan Liu, the senior author of the study, said, "Our work not only elucidates the mechanism behind the emergence of ferroelectricity in binary oxides but also pave the way for innovative advances in the semiconductor technology."
"The advancement of innovative computing methods, such as neuromorphic computing, is closely tied to the development of novel devices and architectures. A primary area of emphasis is ferroelectric materials, which are essential for integration with existing CMOS technology. We demonstrate that ferroelectricity can be engineered in conventional amorphous high-κ dielectrics by simply adjusting the oxygen level during the low-temperature ALD deposition."
"The discovery of emerging ferroelectricity in amorphous binary oxides opens up a new path for non-volatile storage technology solutions, which can avoid the shortcomings of reliability degradation and gate leakage increment in scaling poly-crystalline doped HfO2-based films. Based on the amorphous dielectrics, a non-volatile memory device with low temperature process compatibility, low leakage current, excellent reliability and low operating voltage can be realized."
The presented approach expands the research subject of conventional ferroelectricity to engineer a number of widely used extremely thin binary oxide for logic or memory transistors for future CMOS technology.
More information: Huan Liu et al, Evidence for reversible oxygen ion movement during electrical pulsing: enabler of the emerging ferroelectricity in binary oxides, Materials Futures (2024). DOI: 10.1088/2752-5724/ad3bd5
Provided by Songshan Lake Materials Laboratory