This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Unheard of in structural biology': New enzyme models reveal disease insights
When nucleic acids like DNA or RNA build up in a cell's cytoplasm, it sets off an alarm call for the immune system. Enzymes usually clear these nucleic acids before they cause an issue, but when these enzymes don't work and the immune system gets called in, it can lead to autoimmune and inflammatory diseases.
In a new study published in Structure, Scripps Research scientists present the previously undescribed structure of two of these nucleic acid-degrading enzymes—PLD3 and PLD4. Understanding these enzymes' structures and molecular details is an important step toward designing therapies for the various diseases that arise when they malfunction, which include lupus erythematosus, rheumatoid arthritis and Alzheimer's disease.
"These enzymes are important for cleaning up the cellular environment, and they also set the threshold for what is considered an infection or not," says senior author David Nemazee, Ph.D., professor in the Department of Immunology and Microbiology at Scripps Research. "I'm hoping someday we may be able to help patients based on this information."
Enzymes are proteins that speed up chemical reactions by binding and reacting to specific molecules called substrates. In the case of PLD3 and PLD4, the substrate is a strand of RNA or DNA, which the enzymes break down nucleotide by nucleotide.
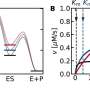
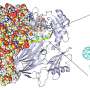
The team used X-ray crystallography to build atomic-scale models of the PLD3 and PLD4 in multiple states or situations, allowing them to examine how their shapes changed over the course of the catalytic reaction. This included when the enzymes were resting, or when they were actively bound to a substrate.
"These models allow us to visualize PLD3 and PLD4 very clearly and with high resolution, so we know exactly how every atom interacts, meaning we can deduce how the enzymes work," says first author Meng Yuan, a staff scientist in the Department of Integrative Structural and Computational Biology at Scripps Research.
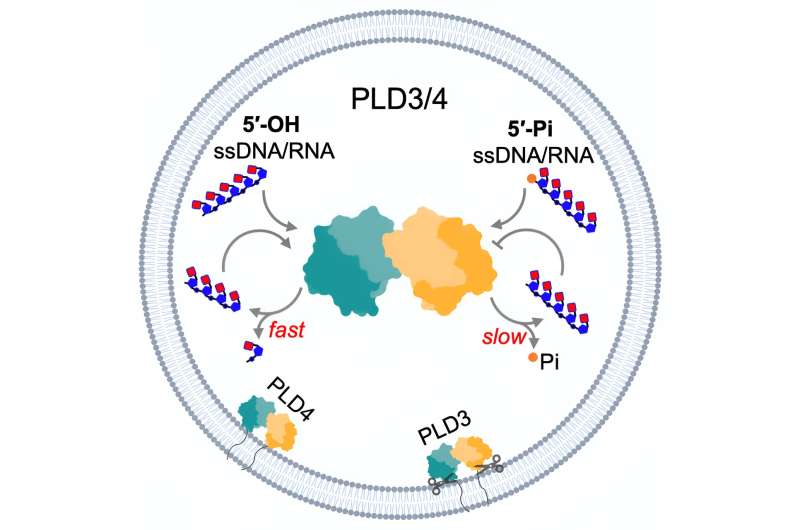
The structural analyses revealed that PLD3 and PLD4 are structurally similar and that they degrade DNA and RNA in a very similar fashion, even though PLD4 is a larger protein. Both enzymes degrade nucleic acids via a two-step process.
"We call this process a two-step catalysis: bite down and release," says Yuan. "In the first step, the enzyme bites down on the DNA strand and separates a single 'brick' or nucleotide from the rest of the strand, and in the second step, it opens its 'mouth' and releases the brick to be recycled."
Because the enzymatic reaction happens so quickly—within milliseconds—researchers needed to use an alternative substrate to visualize the enzymes' structure during catalysis. To do this, they incubated the enzymes together with a molecule that looks very similar to the DNA that the enzyme usually degrades, but that the enzymes degrade much more slowly.
This method uncovered a previously unknown function for one of the enzymes: In addition to biting off nucleotides from single-stranded RNA and DNA, PLD4 also showed phosphatase activity, which means it might also be involved in breaking down DNA's phosphate backbone.
"I think it's amazing that the crystal structure told us about this phosphatase activity," says Nemazee. "To discover new enzymatic activity is unheard of in structural biology. It's only because Meng was able to solve such an amazingly accurate and detailed structure that he could inform us about this extra enzymatic activity that we had no idea about."
After they had elucidated PLD3 and PLD4's usual structure, the researchers examined the structure of variants that are associated with diseases, including Alzheimer's and spinocerebellar ataxia. These analyses revealed that some of these variants had decreased enzymatic capability, while others—including a mutation associated with late-onset Alzheimer's—appeared to be more active.
"Some of our data suggests that one of these Alzheimer's-associated enzyme variants might function better, which was a surprise to me, but it also may be less stable and more easily aggregated," says Nemazee.
The researchers plan to continue investigating the structure and function of these enzymes. Their next steps include exploring possible ways of inhibiting the enzymes in scenarios where they are overactive, and they also plan to investigate the possibility of replacing the enzymes in people who carry non-functional (or non-working) versions.
In addition to Nemazee and Yuan, authors of the study, "Structural and mechanistic insights into disease-associated endolysosomal exonucleases PLD3 and PLD4," were Linghang Peng, Deli Huang, Amanda Gavin, Fangkun Luan, Jenny Tran, Ziqi Feng, Xueyong Zhu, Jeanne Matteson, and Ian Wilson, all of Scripps Research.
More information: Meng Yuan et al, Structural and mechanistic insights into disease-associated endolysosomal exonucleases PLD3 and PLD4, Structure (2024). DOI: 10.1016/j.str.2024.02.019
Journal information: Structure
Provided by The Scripps Research Institute