Selectivity profiling with interaction fingerprints. Credit: Nature Machine Intelligence (2024). DOI: 10.1038/s42256-024-00847-1
Australian researchers, led by Monash University, have invented a new artificial intelligence (AI) tool which is poised to reshape virtual screening in early stage drug discovery and enhance scientists' ability to identify potential new medicines.
Although computational methods within drug discovery are well established, there is an indisputable gap when it comes to novel AI tools capable of rapidly, robustly and cost-effectively predicting the strength of interactions between molecules and proteins—a critical step in the drug discovery process.
The Australian invention "PSICHIC" (PhySIcoCHemICal) brings together expertise at the interface of computing technology and drug discovery to offer an entirely new approach.
Published in Nature Machine Intelligence, the study demonstrates how PSICHIC uses only sequence data, alongside AI, to decode protein-molecule interactions with state-of-the-art accuracy, while eliminating the need for costly and less accurate processes such as 3D structures.
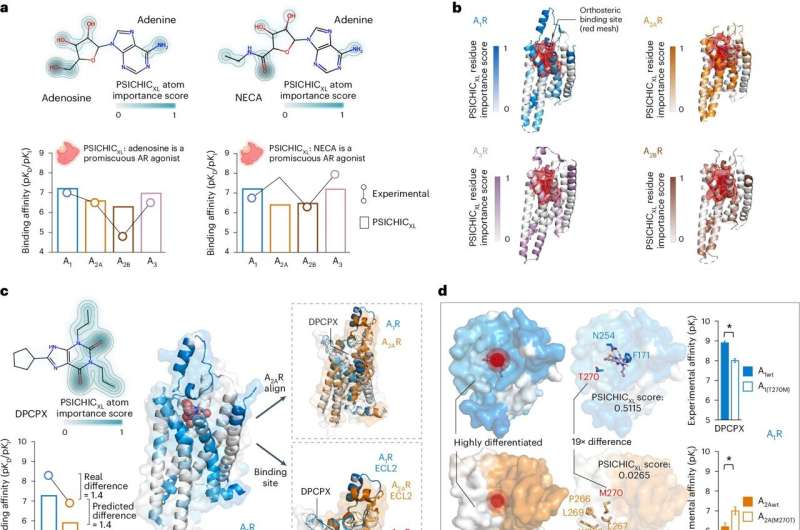
Dr. Lauren May, co-lead author from the Monash Institute of Pharmaceutical Sciences (MIPS), said the team has already demonstrated that PSICHIC can effectively screen new drug candidates and perform selectivity profiling.
"Comparison of experimental and AI predictions of a large compound library against the A1 receptor—a potential therapeutic target for many diseases—demonstrated PSICHIC could effectively screen and identify a novel drug candidate. Moreover, PSICHIC was able to distinguish the functional effects of the compound or, in other words, the way in which the drug might affect our bodies," Dr. May said.
"There is enormous potential for AI to completely change the drug discovery landscape. We foresee PSICHIC reshaping virtual screening and deepening our understanding of protein-molecule interactions."
Data scientist, AI expert and lead author, Professor Geoff Webb from Monash's Department of Data Science and Artificial Intelligence, said while other methods for predicting protein-molecule interactions already exist, they can be expensive and falter in their ability to predict a drug's functional effects.
"The application of AI approaches to enhance the affordability and accuracy of drug discovery is a rapidly expanding area. With PSICHIC, our team has eliminated the need for 3D structures to map protein-molecule interactions, which is a costly and often restrictive requirement," Professor Webb said.
"Instead, PSICHIC identifies the unique 'fingerprints' of specific protein-molecule interactions by applying AI to analyze thousands of protein-molecule interactions, resulting in faster and more effective screening of drug compounds without the need for rendering protein or molecule structures in high-resolution 3D."
Dr. Anh Nguyen, co-lead author from MIPS with strong expertise in AI approaches to drug-receptor interactions, emphasized the importance of these interactions.
"Interactions between molecules and proteins underpin many biological processes, with drugs exerting their intended effects by selectively interacting with specific proteins. There have been significant global efforts to develop new AI-based methods to accurately determine how a molecule might behave when it interacts with its protein target—after all, this is the core building block to making medicines," Dr. Nguyen said.
More information: Huan Yee Koh et al, Physicochemical graph neural network for learning protein–ligand interaction fingerprints from sequence data, Nature Machine Intelligence (2024). DOI: 10.1038/s42256-024-00847-1
The PSICHIC team has made their data, code, and optimized model available to the broader scientific community. Visit www.psichicserver.com for more information.
Journal information: Nature Machine Intelligence
Provided by Monash University