This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biologists take closer look at stress response in cells
A new study from the Zaher Lab at Washington University in St. Louis, published in Molecular Cell, dives into the mechanisms behind the ways cells respond to stress.
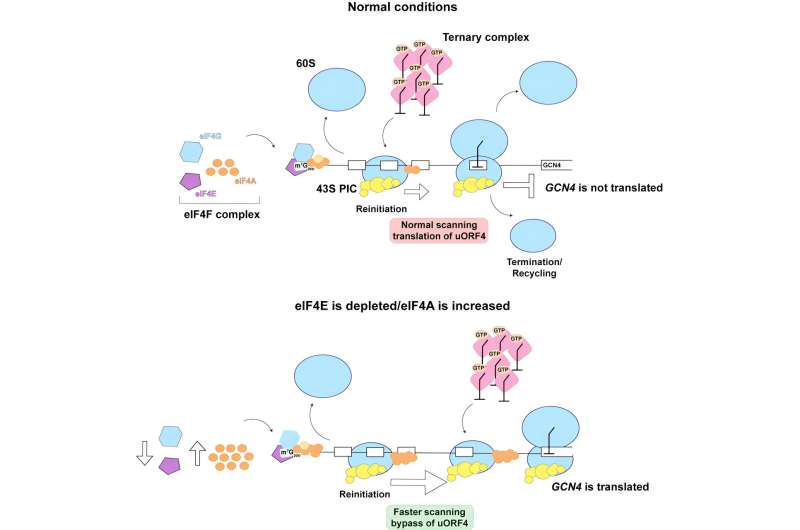
When cells encounter challenges like nutrient shortages, they activate a response known as the integrated stress response (ISR), which produces specific proteins to help the cells survive.
"One of the key proteins produced during the process is a transcription factor called Gcn4," researcher Hani Zaher explained. "What that means is that this protein goes into the nucleus and regulates the expression of hundreds of genes." This Gcn4-mediated regulation results in the production of proteins that help cells adapt to and survive stressful conditions.
In addition to the ISR, cells also rely on the Target of Rapamycin (TOR) pathway to regulate protein production, controlled in part by a protein called eIF4E. This translation initiation factor, which is found in all eukaryotes, is responsible for recruiting the ribosomes—the cell's protein-making machinery—to mRNAs before they can begin translation. The Zaher Lab investigated what happens to protein production when elF4E was removed from the equation in yeast cells. They found that the absence of eIF4E led to an increase in the production of Gcn4.
"How Gcn4 is translated under these conditions was surprising because it is typically produced through a unique translation-based mechanism in response to the ISR process," Zaher said. Notably, they found that translation of Gcn4 occurred through a different mechanism than the typical stress response pathway.
But these findings were only the first part of this research. "We wanted to come up with a model that explained this observation," Zaher said. The model the Zaher Lab developed suggests that the increase in Gcn4 production in the absence of eIF4E may be attributed to changes in the speed at which ribosomes move along genetic instructions. This change in speed may be influenced by increased local concentrations of a factor called eIF4A that help the ribosome traverse along the mRNA.
"We're trying to understand how ribosome speed can be used as a gauge for cellular conditions," Zaher explained.
These findings provide valuable insights into the intricate mechanisms underlying cellular responses to stress and may have implications for understanding why certain cancers alter the concentrations of translation factors such as eIF4E and eIF4A.
More information: Kyusik Q. Kim et al, eIF4F complex dynamics are important for the activation of the integrated stress response, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.04.016
Journal information: Molecular Cell
Provided by Washington University in St. Louis